The study of organometallic chemistry, an area that bridges inorganic and organic disciplines, has found remarkable utility in many diverse applications. Organometallic compounds themselves, which consist of metals bonded to organic groups, can teach us a great deal about fundamental bonding properties. Over the years, we have been examining the catalytic activity of the complexes of Rh, Ir and Pd. While those are expensive “noble metals” and interest globally has turned to catalysis with more “earth abundant” metals, the noble metals still have a great deal of utility because of their unique properties. Lately, we have found that the complexes we have been investigating not only have remarkable catalytic activity but also remarkable biological activity. In a most exciting discovery, we found that some of our compounds show excellent anti-microbial activity (especially against resistant bacteria such as those that cause TB and MRSA) and anti-viral activity (especially against SARS-Cov-2) while showing little to no toxicity to mammalian cells. More below!
Anti-Microbial Activity
Most antibiotics are natural products or derived from them and, so, thinking about organometallic compounds of the transition metals in that role takes a little (lot of) getting used to. We have found that many of the compounds we have been examining for their catalytic chemistry also display remarkable anti-microbial activity. Our ability to change functionality of the complexes at different sites in the molecule has led to a good understanding of structure activity relationships (SARS) and allows us to tune the complexes for different microbes. Examples of those compounds and references to our publications are shown here:
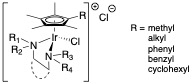
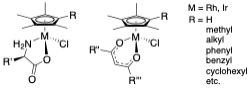
This work has been carried out in collaboration with Prof. Joe Falkinham in the VT Department of Biological Sciences. Most recent papers we have published:
1. Bernier, C. M.; DuChane, C. M.; Martinez, J. S.; Falkinham, J. O.; Merola, J. S., Synthesis, Characterization, and Antimicrobial Activity of Rh III and Ir III N-Heterocyclic Carbene Piano-Stool Complexes. American Chemical Society (ACS): 2021; Vol. 40, pp 1670-1681. DOI:10.1021/acs.organomet.1c00166
2. DuChane, C. M.; Karpin, G. W.; Ehrich, M.; Falkinham, J. O. r.; Merola, J. S.; DuChane, C. M.; Karpin, G. W.; Merola, J. S.; Ehrich, M.; Falkinham, J. O. r., Iridium piano stool complexes with activity against S. aureus and MRSA: it is past time to truly think outside of the box. Royal Society of Chemistry: 2019; Vol. 10, pp 1391-1398. DOI:10.1039/c9md00140a
3. DuChane, C. M.; Brown, L. C.; Dozier, V. S.; Merola, J. S., Synthesis, Characterization, and Antimicrobial Activity of Rh III and Ir III β-Diketonato Piano-Stool Compounds. 2018; Vol. 37, pp 530-538. DOI:10.1021/acs.organomet.7b00742
Anti-Viral Activity
Recognizing the anti-microbial activity of our complexes and being in the middle of a pandemic, the opportunity to test compounds for their anti-viral activity was too good to pass up. Thanks to our colleague James Weger-Lucarelli in the VT Department of Biomedical Sciences, we discovered that different classes of our compounds had very good anti-viral activity against Sars-CoV-2 and other viruses. Again, different functionality allows for tuning the activity of the compounds, an aspect under current investigation. The classes of complexes shown below were tested, but not all showed activity. Only the NHC and acac compounds 2 and 4 did so.
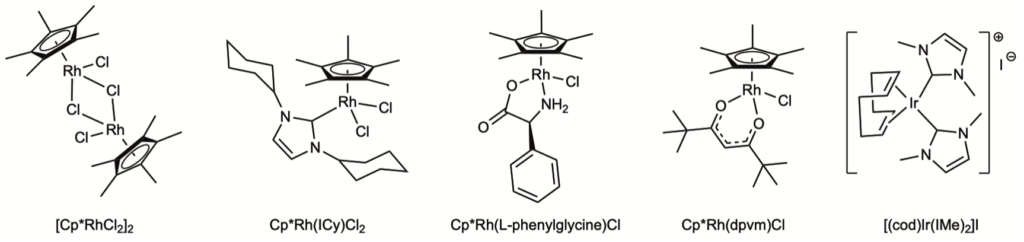
Compounds 2 and 4 in this figure show the highest activity agains Sars-CoV-2
1. Chuong, C.; DuChane, C. M.; Webb, E. M.; Rai, P.; Marano, J. M.; Bernier, C. M.; Merola, J. S.; Weger-Lucarelli, J., Noble Metal Organometallic Complexes Display Antiviral Activity against SARS-CoV-2. Viruses 2021, 13 (6). doi:10.3390/v13060980
Metal Amino Acid Chemistry for Catalysis
Some of our examination of our complexes for biological activity actually began with our investigation into amino acid complexes of metals for their catalytic activity. Amino acids provide a very inexpensive chiral ligand that allow the catalyst to produce chiral products. We have been examining several compounds for a process called asymmetric transfer hydrogenation (ATH). This process produces chiral alcohols the need for which abound in pharmaceuticals, flavors and fragrances. The latest example of our work is shown in the image below.
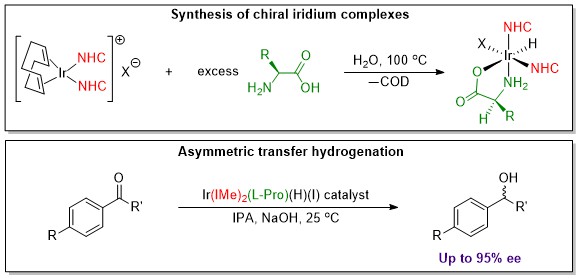
References to some of the systems we have investigated follow:
1. Bernier, C. M.; Merola, J. S., Design of Iridium N-Heterocyclic Carbene Amino Acid Catalysts for Asymmetric Transfer Hydrogenation of Aryl Ketones. Catalysts 2021, 11 (6). doi:10.3390/catal11060671
2. Morris, D. M.; McGeagh, M.; De Pe{~n}a, D.; Merola, J. S., Extending the range of pentasubstituted cyclopentadienyl compounds: The synthesis of a series of tetramethyl (alkyl or aryl) cyclopentadienes (Cp∗ R), their iridium complexes and their catalytic activity for asymmetric transfer hydrogenation. Polyhedron 2014, 84, 120–135. doi:10.1016/j.poly.2014.06.053
